Volume 4 Issue 1
By Emma Hoddle, University of Exeter
Citation
Hoddle, E. (2024) A Paleoecological Investigation into Vegetation Change at Tor Royal, Central Dartmoor as a Basis for Moorland Conservation. Routes, 4(1): 10-26.
Abstract
Through analysis of pollen, charcoal, and spores of coprophilous fungi (SCF), from a core taken from Tor Royal Bog, central Dartmoor, this study reveals a vegetational shift which was driven by a significant event of climatic deterioration (1155-1395 BC), causing widespread change in land use. Statistical analysis revealed the need for multiple baseline conditions from which to make inferences about change. Reconstruction of the vegetational history of the site made it possible to evaluate management strategies with both local bog vegetation and peat accumulation, and regional moorland in mind. This study shows a shift from widespread woodland to a heterogenous landscape of biodiverse bogland vegetation, with pockets of woodland and extensive acid grassland. The role of grazing and burning reveals much of the motivation behind changing vegetation patterns. Peat rewetting and prescribed grazing were noted as management techniques in the face of anthropogenic climate change, as they both promote resilient biodiversity, and encourage the sequestration of carbon.
1. Introduction
Palaeoecology is the study of past long term vegetation change. There is an inherent disconnect in the current discourses of ecological and palaeoecological disciplines (Froys and Willis, 2008), with only 3% of studies concerning conservation management considering timescales longer than a decade (Ormerod, 1999). Short-term ecological studies based on observation alone cannot effectively inform management, as some vegetational change may not be seen for decades (Willis et al., 2005; Grant and Edwards, 2008; Lindbladh et al., 2008). Palaeoecology uses various scientific techniques in order to understand important questions about past climate change (Birks, 2012), using environmental proxies. For example, pollen presence is used as a proxy for vegetation presence on a landscape.
10% of the world’s total blanket mire is located in the British Isles (Yeloff et al., 2006), accounting for nearly 50% of the UK’s terrestrial carbon (Milne and Brown, 1997). Peat has been developing on Dartmoor asynchronously since the beginning of the early Holocene (Fig. 1) (Fyfe and Woodbridge, 2012), making it a nationally significant carbon sink. Effects of climate change as a result of warming and drought can cause vegetation disruption and a lowering of the water table, which could cause peat to erode and decompose. When this occurs, the carbon flux into the atmosphere is increased, contributing to the positive feedback of global warming. TRB has been selected as the study site as it is still accumulating peat today, and has done through time, making it an important carbon sink. Understanding why and how this site has persisted and accumulated organic material through time will help us to gain a better understanding of how to manage peat bogs today.

Figure 1: A map of peatland extent on Dartmoor.
This study aims to understand the effect of palaeoclimatic change on upland ecosystems and peat bogs by carrying out a multi-proxy analysis on a core of peat taken from TRB. Due to the peat’s long history it has accumulated valuable proxies transported to the bog through fluvial and subaerial processes, which can tell us about past vegetation structures. Collection and counting of microfossil remains from within the peat (Blackford, 2000) will allow us to infer how the landscape evolved over time through the layers of sedimentation. This study will try to understand what vegetation change occurred as a result of a climatic wet shift highlighted by Amesbury et al. (2008). Amesbury suggested that this wet shift caused humans to abandon their field systems (termed ‘reaves’), which may have enhanced vegetation change on Dartmoor. Identification of these shifts and an understanding of when or if they have occurred in the past in a certain place, can allow one to understand the impacts of these shifts on vegetation change, and then can be applied to inform inferences about how close to a threshold, and how resilient or vulnerable an ecosystem is (Froyd and Willis, 2008).
Multiple sets of reference conditions (‘baselines’) will be used from which to assess current changes (Froyd and Willis, 2008). Baselines are stable-state conditions, and literature suggests ecosystems often have multiple baselines (Davies and Bunting, 2010; Froyd and Willis, 2008; Willis et al., 2010). It is important to consider multiple baselines to represent the dynamic nature of change over time. This is particularly true where humans have altered the landscape. Human alteration of the landscape can be explored through proxies such as pollen, charcoal, and SCF, which can be used to make inferences about agriculture, burning, and grazing respectively. We may then apply our understanding of how the vegetation changes to contemporary management strategies to promote the biodiversity of the uplands, and prevent peat degradation (Moore and Knowles, 1989), which would be a catalyst for global warming.
2. Study Area and Methods
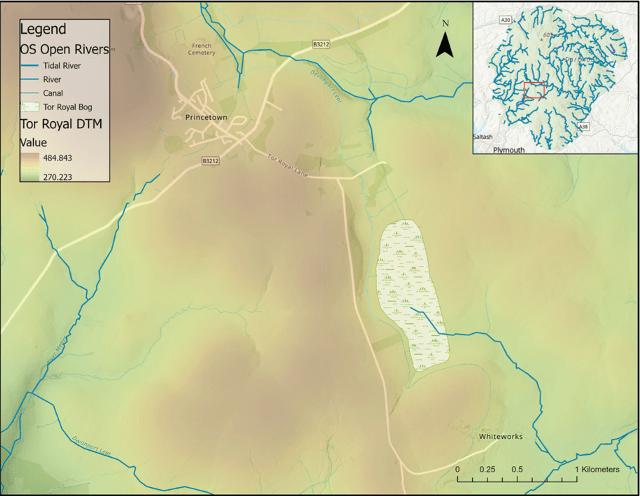
Figure 2: A map of TRB study site, with a Digital Terrain Model (DTM) to portray local topography, and local rivers (OS Open Rivers).
A 595 cm core was extracted from TRB (Figs. 2, 3), (50.53576° N, 3.97099° W) Central Dartmoor, using a Russian corer. TRB is an ombrotrophic raised upland mire, with Ericaceous shrubs, Tricophorum cespitosus, Eriophorum spp. and Sphagnum mosses (West, 1997). The site was chosen due to the health of the bog and the remoteness from the reave systems (Amesbury, et al. 2008), thus it is less likely to be interfered with by sensitive changes of human occupation and land use. The core was extracted close to previous cores by Amesbury et al. (2008) and West (1996), allowing a chronology to be constructed by cross correlating them in reference to stratigraphical features and depth. 0.5m sections were taken, and, to closely study the climatic shift, samples were extracted every 12.5 cm from 220-288 cm, and every 6.25 cm from 295-300 cm.
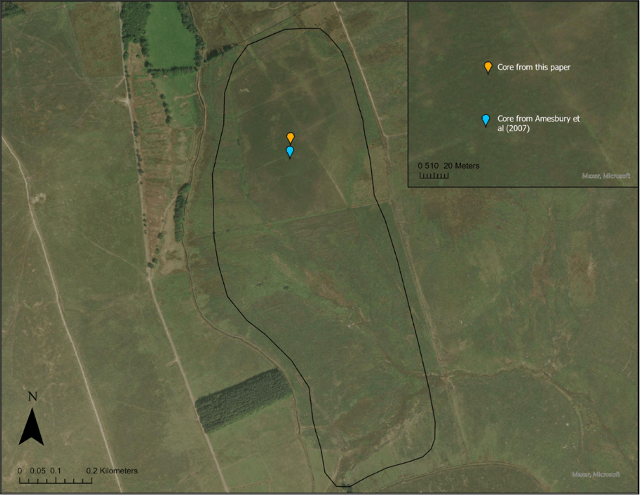
Figure 3: A map showing the location of the core from this paper, and the location of Amesbury’s core (2008).
Preparation for microscope analysis through acetolysis and density separation was done at the University of Exeter. Samples were spiked with Lycopodium tablets at a concentration of 20,848 per sample and mounted on a slide. Pollen, SCF and charcoal were counted to 200 Lycopodium. Pollen was identified using modern reference material archived in a pollen reference collection at the University of Exeter, and images and descriptions from the Global Pollen Project (Martin and Harvey, 2017). Spore identification was conducted using published descriptions and fungal keys (van Geel et al., 2003). Features such as grain number, size, shape, surface structures, and internal detail were examined to obtain high taxonomic resolution and accuracy (Weber, 1998). Detrended Correspondence Analysis was done using Past4 software, on the percentage pollen assemblage data from both this study and data from West (1998), to identify ecological differences through time.
3. Results and Discussion
3.1 Chronology
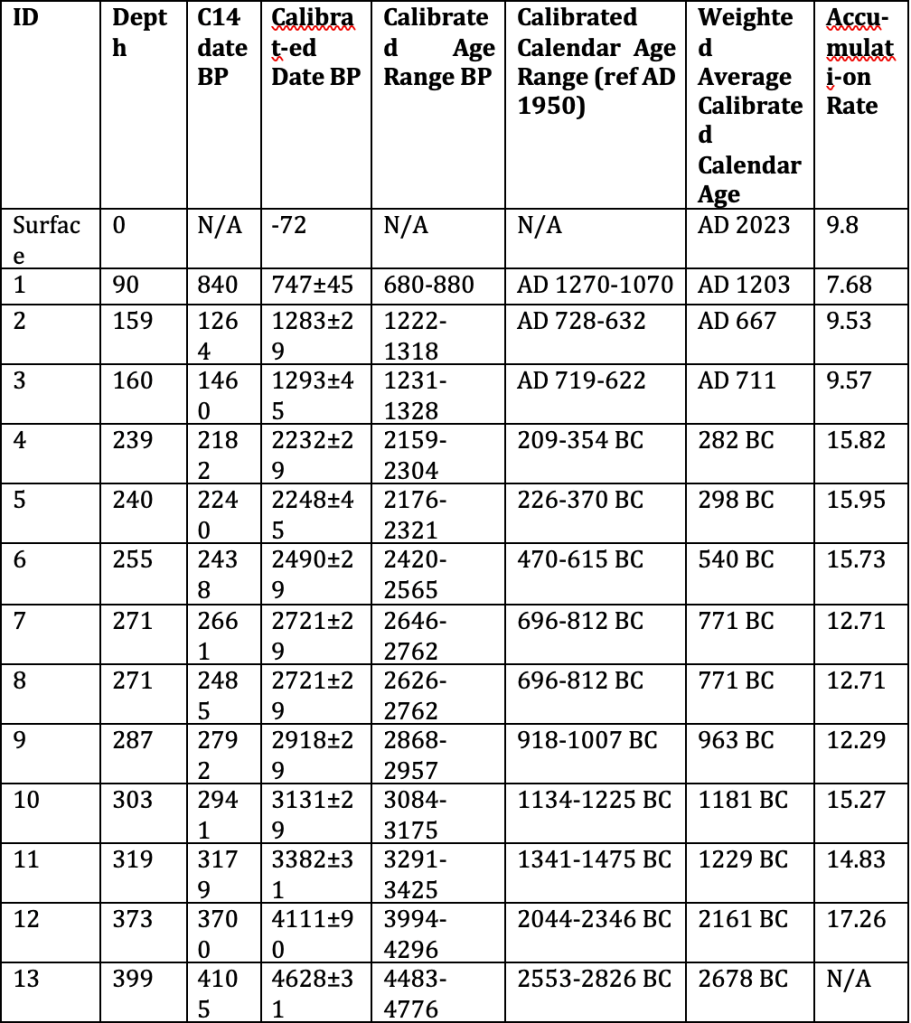
Table 1: Radiocarbon ages (14C) from Amesbury (2008) and West (1997), and their calibrated ages (cal yr BP), and accumulation rates from this study.
An age-depth model was constructed (Fig. 4) using the radiocarbon dates from Amesbury et al. (2008) and West (1997) using Clam (Blaaw, 2010), and calibrated using an IntCal20 radiocarbon calibration curve (Reimer et al., 2020). A smooth spline was used to calculate the chronology, update the previous estimates of the core, and provide appropriate estimates for the core collected in this study.
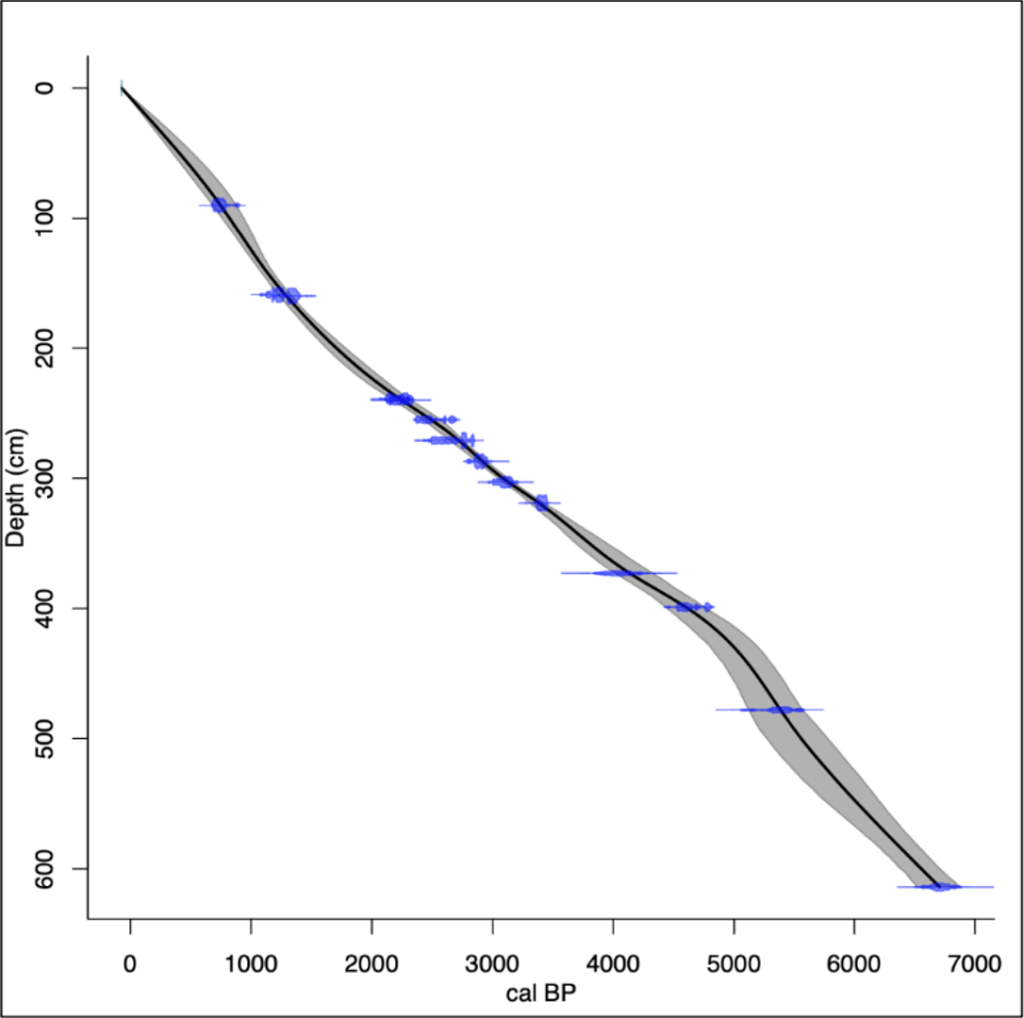
Figure 4: A Bayesian Age Depth Model for TRB. Blue plots represent the radiocarbon dates from both West (1997) and Amesbury (2008). Lines show the probability distribution for each calibrated date. System initiation is seen around ~4800 BC, with an average accumulation rate of 11.04cm/yr.
3.2 Development of the Mire (Stratigraphical Description)
Stratigraphy has little variance throughout the core but increased in darkness and compactness with increasing depth (Figs. 5, 6). Macroscopic plant material became finer and more acute with increasing depth. The water table at time of coring was 38 cm, the acrotelm layer of active decomposition (the top layer of peat) is relatively small, comprised of plenty of root material. This high-water table is an indication that the bog is still accumulating, plant matter in the acrotelm is likely to only partially decompose before it subsides under the water table into the catotelm. Degree of humification increases down the core (Amesbury et al., 2007; West, 1996). Initiation of the mire at TRB occurred ca. 4800 BC, and developed as a minerotrophic mire until ca. 1500 BC (West, 1997). The Fen-Bog Transition was identified at the time of the wet shift through analysis of geochemical signals (West, 1997). Increased effective precipitation as a result of the wet shift caused an increase in the accumulation rate of the bog, from an average of 11.04 cm/yr to 15.2 cm/yr (Table 1). The rate of increase was enough to cause a separation between the surface and the water table, initiating a pioneer oligotrophic bog stage (Hughes and Barber, 2003), after which a Sphagnum-dominated (Fig. 7) ombrotrophic raised mire developed. Evidence from this study found a healthy and persistent bog system, characterised by a high-water table, thick vegetation cover, and little human disturbance (Figs. 6, 9).

Figure 5: A stratigraphic diagram the appearance of the peat core showing. from this study.

Figure 6: Images of the peat from different sections of the core, showing its colour and visible detritus.

Figure 7: Image of Sphagnum moss found at TRB. (Image: Wikimedia)
3.3. Ecological Baseline
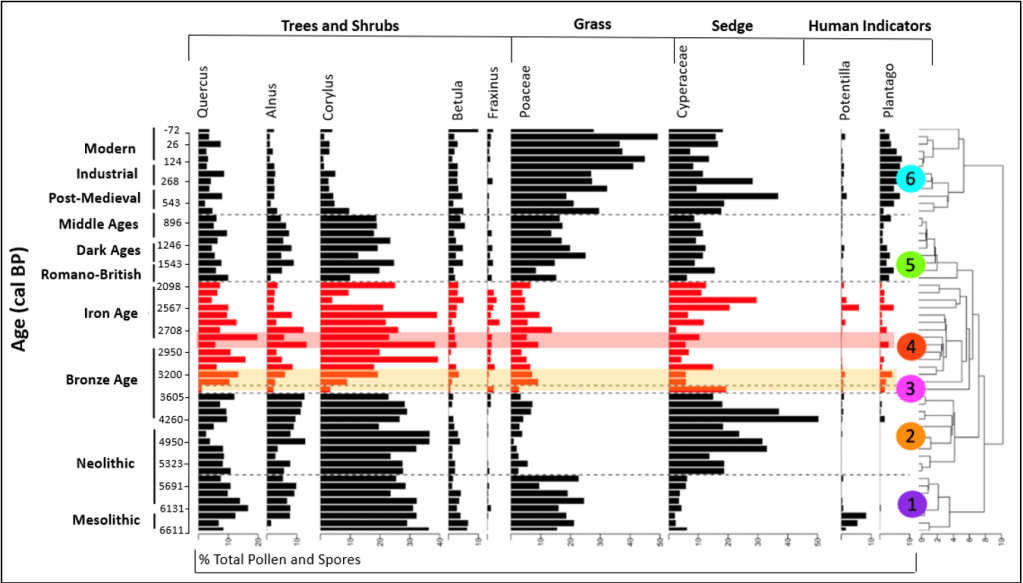
Figure 8: Stratigraphic diagram of the pollen taxa from Tor Royal Bog in this study (red/DCA-3 and 4) and data obtained from West (1997) in black. The zones identified from the DCA analysis are labelled on the right, identified using the Bray-Curtis method of dissimilarity to quantify the differences in the species composition between the samples, using the total pollen concentration per cm3 data. Zones are different colours to help distinguish them. Orange shading shows the wet shift, and red shading highlights the Bronze-Iron age transition.
Microfossil analyses show an ecological shift from an upland environment, in the form of a change from significant woodland cover, to a fragmented landscape of open woodland, heathland, and grassland pasture (Fig. 8). Statistical analyses identified a sample reflecting an abrupt change in vegetation at 320 cm core depth (DCA- 3), ca. 1447 BC, inferred through a sharp change in pollen concentrations. The wet shift, and the ecological shift in this this study can be matched up with confidence.
Statistical analysis has revealed two zones prior to the onset of the wet shift in 1395-1155 BC (Fig. 8). The evidence presented provides an estimate for the state of the upland prior to significant human disturbance (DCA-1), where human activity was limited (Fyfe et al., 2003). Then, DCA-2 shows that people started to influence the environment, an increase of Cyperaceae (sedge), and a decline in woody taxa such as Quercus and Betula, could reflect forest clearance. There is also plenty of archaeological evidence of communities on Dartmoor at this time (Caseldine, 1999). Due to the extensive history of human involvement on the uplands of Dartmoor, this study uses both baselines (before and after human influence) to infer change, as they provide a fuller picture of its legacy.
3.4. Vegetation Change
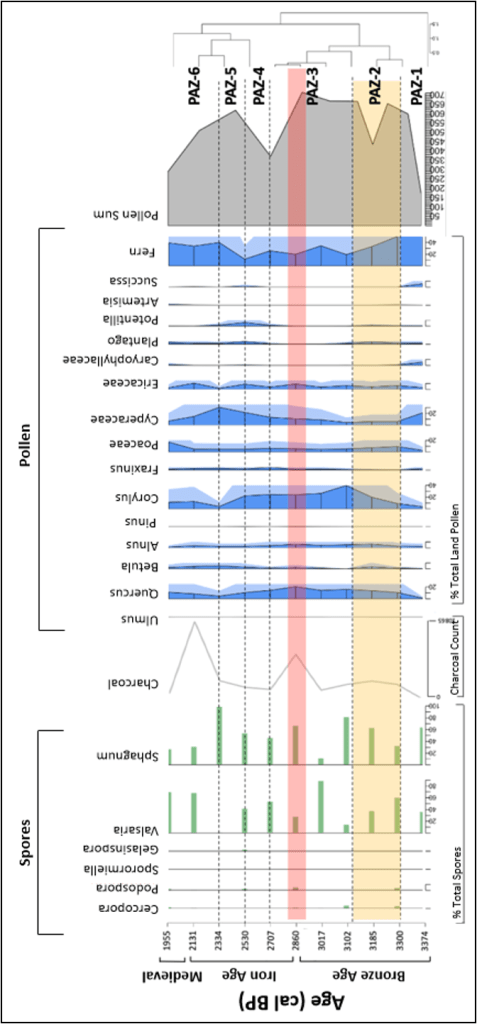
Figure 9: Summary diagram of the most abundant spore and pollen taxa recorded from the sediments of Tor Royal Bog. Spores are presented as percentage of total spores, pollen is presented as a percentage of the total land pollen, and charcoal expressed as count data (i.e. raw count). Highlighted yellow zone shows the wet shift identified in Amesbury et al. (2008), and the red highlighted section shows the Bronze-Iron age transition. Pollen Assemblage Zones (PAZ) are marked on the right, identified using the community ecology package ‘vegan’ and adjusted by eye to group zones of similarity.
| Zone | Pollen, SCF, and Charcoal Characteristics | Inferred Change |
| PAZ-1 | Woodland taxa increase, increase in sedge and prevalence of human indicators. Majority fern (8,756/cm cm3 and Ericaceae present. Low Sphagnum concentrations, with Cercophora and Podospora present. Charcoal levels low. | Significant woodland retreat followed by recovery, with persistence of heathland. Evidence of increased grazing. Little evidence of clearing through burning. |
| PAZ-2 | Decline in Fern species (-20%), accompanied with lower diversity of herbaceous taxa. Ericaceae persists. Woodland taxa experience subsequent increase. Plantago increasing. Presence of Podospora, Sphagnum concentrations increase (to 3856/cm3). Steady increase in charcoal. | Recovery of woodland, increase in Alnus suggests more moist conditions. Grassland areas decline, heathland persists. Increasing evidence of human presence on the upland, is indicated by Plantago and Potentilla, and some evidence of grazing. Increasing Sphagnum concentrations indicate increasingly moist climate. Charcoal concentrations have not peaked over a regional background level. |
| PAZ-3 | Fluctuating levels of fern (34-19%), but Ericaceae remains stable. Increasing Poaceae and Cyperaceae. Stable woodland taxa concentrations with increasing Quercus. Spike in Valsaria (~22,500cm3), and increasing Sphagnum concentrations. Presence of Podospora and Cercophora. Large peak in charcoal concentrations (6,615 fragments). | Heathland experiences some change, some evidence for management of the heathland with fire, a peak in the charcoal concentrations coincides with an increase in Ericaceae. Corylus dominated open scrubland seems to be widespread. Vegetational indicators of human presence are lower, but spore evidence of grazing is significant. Increasing Sphagnum concentrations indicate development of peat in moist conditions. |
| PAZ-4 | Lowest fern concentrations (11.6%), but Ericaceae persists. Increase in both Plantago and Potentilla. Fern decreases. Decline in woodland taxa, particularly Quercus and Corylus. Increased Fraxinus concentrations. Decrease in the sum of coprophilous spores. Low charcoal concentrations. | Fern-dominated heathland in decline, and woodland retreat. Significant human presence, lack of charcoal evidence does not support human-induced woodland clearance. Small areas of scrubland. Lower evidence of grazing. |
| PAZ-5 | Recovery of Fern pollen (39.8%). Large increase in Cyperaceae (30.3%). Further decline in woodland taxa. Presence of Plantago and Potentilla. Largest increase in Sphagnum (120,292/cm3), presence of a variety of coprophilous fungi. Slight increase in charcoal. | Heathland recovers again. Coupled increase in sedge (Cyperaceae) and Sphagnum implies a local increase in wetness. Grassland persists. Further retreat of woodland. Increased evidence of grazing. |
| PAZ-6 | Decreasing fern concentrations but increase in Ericaceae. Peak of Poaceae concentrations (17%, 5107.8/cm3). Persistent record of Plantago. Woodland taxa remain low. Sphagnum in decline, but a variety of coprophilous spores present. Increase in charcoal concentrations (10,865 at 232.5cm). Cyperaceae concentrations decline. | Persistence of heathland. Woodland remains in retreat, perhaps its resurgence is prevented through human influence, inferred from heightened levels of charcoal. Local grazing evident due to coprophilous fungi and the increase of grass indicating pasture. |
Amesbury et al. (2008) identified the wet shift as a prime contributing factor to the abandonment of the reaves ca. 1500-1200 BC. There is some evidence of woodland regeneration at this time (Fig. 9) (Corylus, Quercus) compared to PAZ-1, suggesting retreat of humans from the uplands. However, there is increased prevalence of human indicator vegetation Plantago and Potentilla in PAZ-4. Further, the SCF record shows some local evidence of grazing, as SCF grow on animal dung, so would increase in concentration when animals are inhabiting. Also, there are a few significant peaks in charcoal, alluding to continued human presence after the wet shift (Fig. 9). Charcoal is produced during incomplete combustion, and tiny fragments may be transported by wind and fluvial processes to settle in the bog (Fig. 10). A higher concentration of microcharcoal would allude to a more extensive fire event, or more frequent, smaller events. Human persistence on Dartmoor is likely due to a purposeful adaptive strategy from upland communities resulting from a deterioration of the climate (Tipping et al., 2008). Soil-hydrological change and increased waterlogging made crop failure far more likely. Our results show an increase in variety of SCF throughout this period (PAZ-2), which could represent the presence of local grazing animals (Gill et al., 2013). A small increase in Poaceae concentrations during ca. 1500-1200 BC occurs, representing the presence of pasture, and decrease in ferns could be related to the prevalence of trampled land in sites of pasture (Blackford et al., 2006). This study finds a partial abandonment of the reaves, some areas with increased agricultural activity and expansion due to the change in growing season and the increased risk of crop failure.

Figure 10: A schematic diagram showing the process by which environmental processes by which charcoal, SCF, and pollen get transported to the peat bog.
3.5. Implications for Conservation Management
TRB has experienced slower peat accumulation over time (Table 1), thus should be observed to ensure that the system can support itself under anthropogenic climate change. There must be prevention of formation of erosional features (Bragg and Tallis, 2001) so that drainage is limited, and carbon is not lost (Evans et al., 2006). Lowering of water tables needs to be managed, as this will alter species composition of Sphagnum towards plant species such as Molinia caerulea, which will lower the input of carbon into long-term stores due to higher decomposition rates of these species (Thorman et al., 1999). Prompt rewetting of the peat would be a sound strategy (Günther et al., 2020) if the water table at TRB were to subside, to quickly re-initiate sinking of carbon.
These results revealed historical management of heathland. Burning was used to promote the regrowth of young heather shoots, fertilising the soil with the ash to increase the productivity of the area (Davies et al., 2022). Management should therefore induce the instability from which the heathland has adapted, through evolutionary enhancement of seed germination in response to smoke (Bargmann et al., 2014), supporting the continued use of ‘swaling’ on Dartmoor.
4. Conclusion
4.1. Summary and Significance
This study has identified a significant shift in regional vegetation assemblage, and analysis of microfossil evidence confirmed that this occurred as a result of human and climatic perturbation. This has furthered current understandings of past human-environment relationships and regional climate change, implementing refined Bayesian statistics, and sophisticated multi-proxy lab analyses, significantly contributing to the synthesis of palaeoenvironmental data into conservation research. The accumulation history of TRB revealed a long-term carbon sink, with accumulation of carbon since 4628±31 cal BP. This is a significant contribution to the global carbon cycle, by storing large amounts of carbon. This research has identified viable management strategies to ensure this as a carbon sink in the future under global warming, and may be implemented in any degraded peatland where the full ecological history is understood.
4.2. Evaluation and Future Directions
The dates used in this study from West (1997) are of low resolution, hindering the chronological control. Future studies should undertake further radiocarbon dating in the site, to further resolve the chronology. This would help us to better understand timings and durations of change. Spatial extents of change can also be further investigated through coring of more sites around Dartmoor, particularly in areas with thickest peat. This would create a fuller picture of ecological change on Dartmoor throughout the Holocene.
Acknowledgements
I would like to thank first and foremost Dr Dunia Urrego for her invaluable guidance and support throughout this entire process. Her kindness and understanding have made it far more manageable and enjoyable. My gratitude extends to Dr Tom Roland, who has provided me with irreplaceable advice throughout, and was responsible for organising the excavation of the core. The team in the lab; Ang, Joana, Anggi, and Felix made the process of lab work far more enjoyable, their encouraging words and smiles were greatly appreciated.
References
Amesbury, M.J. et al. 2008. “ronze age upland settlement decline in southwest England: Testing the Climate Change Hypothesis. Journal of Archaeological Science, 35(1): 87–98. Available at: https://doi.org/10.1016/j.jas.2007.02.010.
Bargmann, T., Måren, I.E. and Vandvik, V. 2014. Life after fire: Smoke and ash as germination cues in ericads, herbs and graminoids of Northern Heathlands. Applied Vegetation Science, 17(4): 670–679. Available at: https://doi.org/10.1111/avsc.12106.
Blaauw, M. 2010. Methods and code for ‘classical’ age-modelling of radiocarbon sequences. Quaternary Geochronology, 5(5): 512–518. Available at: https://doi.org/10.1016/j.quageo.2010.01.002.
Blackford, J. 2000. Palaeoclimatic records from peat bogs. Trends in Ecology & Evolution, 15(5): 193–198. Available at: https://doi.org/10.1016/s0169-5347(00)01826-7.
Blackford, J.J. et al. 2006. Mid-holocene environmental change at Black Ridge Brook, Dartmoor, SW england: A new appraisal based on fungal spore analysis. Review of Palaeobotany and Palynology, 141(1-2): 189–201. Available at: https://doi.org/10.1016/j.revpalbo.2006.03.009.
Bragg, O.M. and Tallis, J.H. 2001. The sensitivity of peat-covered upland landscapes. CATENA, 42(2-4): 345–360. Available at: https://doi.org/10.1016/s0341- 8162(00)00146-6.
Caseldine, C.J. 1999. Archaeological and environmental change on prehistoric Dartmoor— current understanding and future directions. Journal of Quaternary Science, 14(6): 575–583. Available at: https://doi.org/10.1002/(sici)1099-1417(199910)14:6<575::aid- jqs491>3.0.co;2-g.
Dark, P. 2006. Climate deterioration and land-use change in the first millennium BC: Perspectives from the British palynological record. Journal of Archaeological Science, 33(10): 1381–1395. Available at: https://doi.org/10.1016/j.jas.2006.01.009.
Evans, M., Warburton, J. and Yang, J. 2006. Eroding blanket peat catchments: Global and local implications of upland organic sediment budgets. Geomorphology, 79(1-2): 45–57. Available at: https://doi.org/10.1016/j.geomorph.2005.09.015.
Fleming, A. 1988. The Dartmoor Reaves: Investigating Prehistoric Land Division, illustrated, Batsford: Indiana University.
Froyd, C.A. and Willis, K.J. 2008. Emerging issues in Biodiversity & Conservation Management: The need for a palaeoecological perspective. Quaternary Science Reviews, 27(17-18): 1723–1732. Available at: https://doi.org/10.1016/j.quascirev.2008.06.006.
Fyfe, R.M. and Woodbridge, J. 2012. Differences in time and space in vegetation patterning: Analysis of pollen data from Dartmoor, UK. Landscape Ecology, 27(5): 745–760. Available at: https://doi.org/10.1007/s10980-012-9726-3.
Fyfe, R.M., Brown, A.G. and Coles, B.J. 2003. Mesolithic to Bronze Age vegetation change and human activity in the Exe Valley, Devon, UK. Proceedings of the Prehistoric Society, 69: 161–181. Available at: https://doi.org/10.1017/s0079497x00001298.
Grant, M.J. and Edwards, M.E. 2007. Conserving idealized landscapes: Past history, public perception and future management in the New Forest (UK). Vegetation History and Archaeobotany, 17(5): 551–562. Available at: https://doi.org/10.1007/s00334-007- 0100-3.
Günther, A. et al. 2020. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nature Communications, 11(1). Available at: https://doi.org/10.1038/s41467-020-15499-z.
Hughes, P.D. and Barber, K.E. 2003. Mire development across the fen-bog transition on the Teifi floodplain at Tregaron bog, Ceredigion, Wales, and a comparison with 13 other raised bogs. Journal of Ecology, 91(2): 253–264. Available at: https://doi.org/10.1046/j.1365-2745.2003.00762.x.
Johnston, R. 2005. Pattern without a plan: Rethinking the bronze age coaxial field systems on Dartmoor, south-West England. Oxford Journal of Archaeology, 24(1): 1–21. Available at: https://doi.org/10.1111/j.1468-0092.2005.00222.x.
L, D.A. and J, B.M. 2010. Applications of Palaeoecology in Conservation. The Open Ecology Journal, 3(2): 54–67. doi:https://doi.org/10.2174/1874213001003020054.
Lindbladh, M. et al. 2008. Close anthropogenic control of fagus sylvatica establishment and expansion in a Swedish protected landscape – implications for forest history and conservation. Journal of Biogeography, 35(4): 682–697. Available at: https://doi.org/10.1111/j.1365-2699.2007.01813.x.
Martin, A. C. and Harvey, W. J. 2017. The Global Pollen Project: a new tool for pollen identification and the dissemination of physical reference collections. Methods Ecol Evol, 8: 892–897. doi:10.1111/2041-210X.12752.
Milne, R. and Brown, T.A. 1997. Carbon in the Vegetation and Soils of Great Britain. Journal of Environmental Management, 49(4): 413–433. doi:https://doi.org/10.1006/jema.1995.0118.
Moore, T.R. and Knowles, R. 1989. The influence of water table levels on methane and carbon dioxide emissions from peatland soils. Canadian Journal of Soil Science, 69(1): 33–38. Available at: https://doi.org/10.4141/cjss89-004.
Ormerod, S.J. 1999. Communicating the value of ecology. Journal of Applied Ecology, 36(6): 847. doi:10.1046/j.1365-2664.1999.00474.x.
Reimer, P.J., Austin, W.E., Bard, E., Bayliss, A., Blackwell, P.G., Ramsey, C.B., Butzin, M., Cheng, H., Edwards, R.L., Friedrich, M. and Grootes, P.M. 2020. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon, 62(4), pp.725-757.
Thormann, M.N., Szumigalski, A.R. and Bayley, S.E. 1999. Aboveground peat and carbon accumulation potentials along a bog-fen-marsh wetland gradient in Southern Boreal Alberta, Canada. Wetlands, 19(2): 305–317. Available at: https://doi.org/10.1007/bf03161761.
Tipping, R. et al. 2008. Response to late bronze age climate change of farming communities in North East Scotland. Journal of Archaeological Science, 35(8): 2379–2386. Available at: https://doi.org/10.1016/j.jas.2008.03.008.
van Geel, B. et al. 2003. Environmental reconstruction of a Roman period settlement site in Uitgeest (the Netherlands), with special reference to coprophilous fungi. Journal of Archaeological Science, 30(7): 873–883. Available at: https://doi.org/10.1016/s0305- 4403(02)00265-0.
Weber, R.W. 1998. Pollen identification. Annals of Allergy, Asthma & Immunology, 80(2): 141–148. Available at: https://doi.org/10.1016/s1081-1206(10)62947-x.
Weir, J.R., Scasta, J.D. and Davies. 2022. in Global application of prescribed fire. Boca Raton, FL: CRC Press.
West, S., Charman, D.J. and Grattan, J. 1996. Palaeoenvironmental investigations at Tor Royal, central Dartmoor. The Quaternary of Devon and East Cornwall: Field Guide. London: Quaternary Research Association, pp.62-80.
Willis, K. et al. 2005. Providing baselines for Biodiversity Measurement. Trends in Ecology & Evolution, 20(3): 107–108. Available at: https://doi.org/10.1016/j.tree.2004.12.003.
Yeloff, D.E. et al. 2005. Blanket peat erosion and sediment yield in an upland reservoir catchment in the southern pennines, UK. Earth Surface Processes and Landforms, 30(6): 717–733. Available at: https://doi.org/10.1002/esp.1170.
#Write for Routes
Are you 6th form or undergraduate geographer?
Do you have work that you are proud of and want to share?
Submit your work to our expert team of peer reviewers who will help you take it to the next level.
Related articles
